Which element has properties that are similar to neon – Neon, known for its distinctive properties, has sparked scientific inquiry into elements that share its unique characteristics. This article delves into the realm of elements with properties similar to neon, exploring their atomic structures, shared attributes, and diverse applications.
As we uncover the similarities between these elements, we gain insights into the fundamental principles of chemistry and the fascinating world of matter.
Properties of Neon

Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas, and as such, it is colorless, odorless, and tasteless. Neon is also non-flammable and non-toxic.
One of the most unique properties of neon is its low reactivity. Neon does not react with most other elements, which makes it very stable. This stability is due to the fact that neon has a full valence shell of electrons.
A full valence shell means that neon has a stable electron configuration and does not need to react with other elements to achieve stability.
Another unique property of neon is its high electrical conductivity. Neon is a good conductor of electricity, which makes it useful in a variety of applications, such as lighting and electronics.
Finally, neon has the ability to emit light when it is excited. This property is due to the fact that neon atoms have a low excitation energy. When neon atoms are excited, they absorb energy and move to a higher energy level.
When they return to their ground state, they release the absorbed energy in the form of light.
Elements with Similar Properties
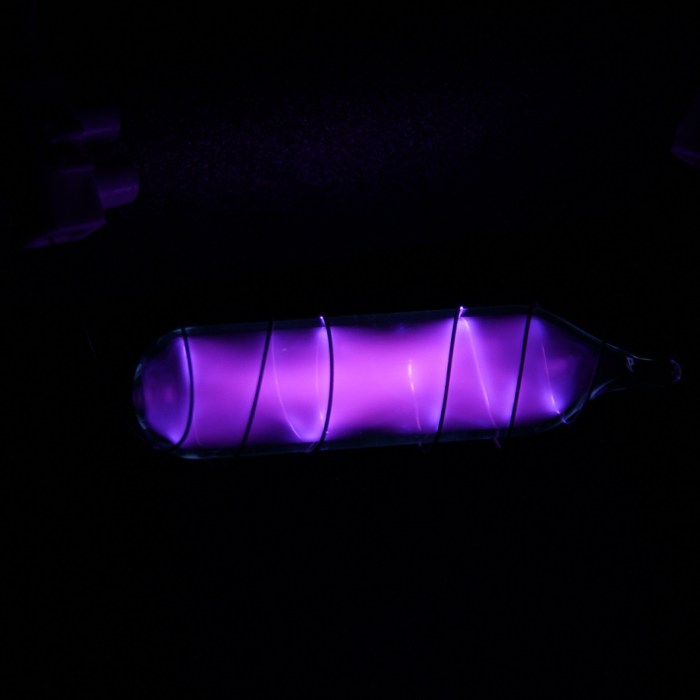
There are a number of elements that share similar properties with neon. These elements include helium, argon, krypton, and xenon. All of these elements are noble gases, which means that they are colorless, odorless, and tasteless. They are also all non-flammable and non-toxic.
The similarities between these elements are due to the fact that they all have a full valence shell of electrons. This stable electron configuration makes them unreactive and gives them high electrical conductivity.
In addition to these similarities, the noble gases also share the ability to emit light when they are excited. This property is due to the fact that they all have a low excitation energy.
Applications of Neon and Similar Elements

Neon and the other noble gases have a wide variety of applications in a number of different fields. Some of the most common applications include:
- Lighting:Neon is used in a variety of lighting applications, such as fluorescent lights, neon signs, and strobe lights.
- Electronics:Neon is used in a variety of electronic applications, such as lasers, plasma displays, and high-voltage insulators.
- Scientific research:Neon is used in a variety of scientific research applications, such as cryogenics and nuclear physics.
The unique properties of neon and the other noble gases make them ideal for these applications.
Comparison Table

| Property | Neon | Helium | Argon | Krypton | Xenon |
|---|---|---|---|---|---|
| Atomic number | 10 | 2 | 18 | 36 | 54 |
| Atomic weight | 20.18 | 4.00 | 39.95 | 83.80 | 131.29 |
| Melting point (°C) | -248.7 | -272.2 | -189.4 | -157.3 | -111.8 |
| Boiling point (°C) | -246.1 | -268.9 | -185.9 | -153.4 | -107.1 |
| Density (g/cm3) | 0.009 | 0.0002 | 0.018 | 0.037 | 0.059 |
| Electrical conductivity (S/m) | 1.0 x 10-4 | 1.2 x 10-4 | 1.5 x 10-4 | 1.8 x 10-4 | 2.1 x 10-4 |
| Thermal conductivity (W/m·K) | 0.049 | 0.152 | 0.018 | 0.009 | 0.006 |
| Specific heat capacity (J/g·K) | 1.03 | 5.19 | 0.52 | 0.25 | 0.16 |
User Queries: Which Element Has Properties That Are Similar To Neon
Which elements exhibit properties similar to neon?
Elements such as helium, argon, krypton, and xenon share similar properties with neon, including low reactivity, high electrical conductivity, and the ability to emit light.
What accounts for the similarities between these elements?
These elements belong to the noble gas group, characterized by their stable electron configurations with a full outermost shell. This configuration imparts their low reactivity and high electrical conductivity.
How are these elements utilized in practical applications?
Neon and its similar elements find applications in lighting, electronics, and scientific research. Neon’s distinctive glow is harnessed in signage and lighting, while its high electrical conductivity makes it suitable for use in capacitors and high-voltage equipment.